News Release
News ReleaseVinBrain to launch AI-centric solutions to save lives and advance precision care at RSNA 2023
Vingroup
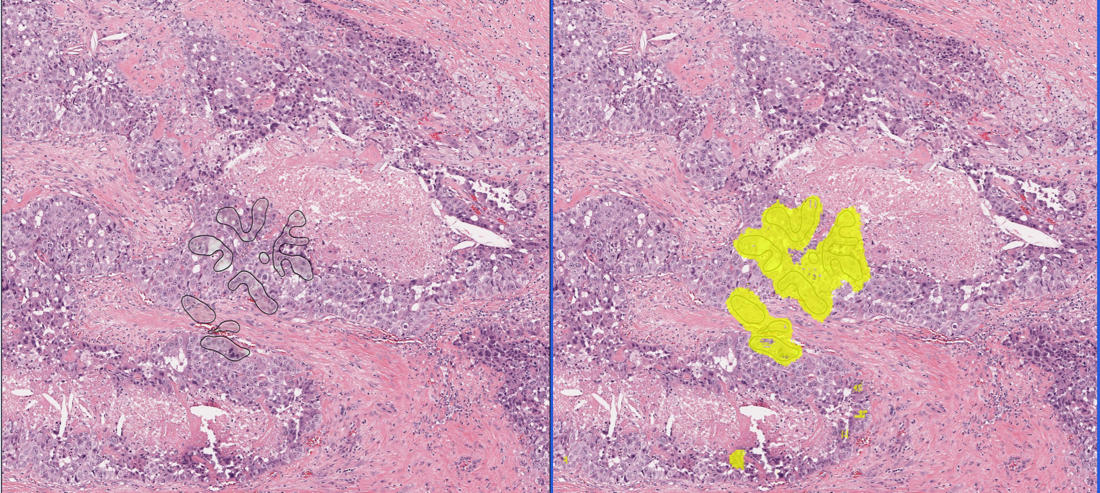
CHICAGO, US - Media OutReach - 4 December 2023 - VinBrain, a leading AI HealthTech company based in Vietnam, funded by Vingroup has joined in the largest medical meetings and exhibition of North America, RSNA 2023. Unveiling two impactful AI-centric solutions during the event - DrAid™ Enterprise Data Solution: Centralization, Transformation, Intelligence; and DrAid™ Oncology Diagnosis and Treatment, VinBrain takes major steps forward in innovation, aiming to save more lives and advance precision care for everyone. RSNA 2023: Leading through Change in AI for diagnosis, treatment, care, interoperability The Technical Exhibits within the 109th Scientific Assembly and Annual Meeting Radiological Society of North America (RSNA), IL, USA have ended after four immersive days, November 26 to 29, of buzzing and breaking advancements across many healthcare subspecialties. Proud to be one of the 113 first-time RSNA exhibitors, VinBrain marks the first time a Vietnamese AI HealthTech start-up has participated in this biggest global Healthcare conference. “VinBrain's evolving product portfolio of AI solutions is being rapidly adopted by physicians,” said Prof. Dr. Gregory Moore, Associate Fellow Center for Artificial Intelligence and Medical Imaging (AIMI) at Stanford University School of Medicine, former Vice President of Microsoft Health global and Google Cloud Healthcare. VinBrain's presence at RSNA goes beyond mere company introduction; it's about learning from and aligning with fellow attendees in our shared mission to deliver high-quality diagnostic radiology care more efficiently and safely. VinBrain gained attention with new game-changers for radiologic AI and streamlining hospital operations. In the exclusive launch at 10:30 am (CT), November 27, the company has debuted two new game-changer solutions, encompassing DrAid™ Enterprise Data Solution: Centralization, Transformation, Intelligence; and DrAid™ Oncology Diagnosis and Treatment (D&T). Expediting interoperability and streamlining, DrAid™ Enterprise Data Solution (EDS) is hoped to lead the healthcare transformation towards precision care. EDS brings a knowledge engine based on big data whose core is “to transfigure massive medical raw data into actionable knowledge and insights”, said Steven Truong, Founder & CEO VinBrain. EDS is capable of auto-generating reports multilingually in more than 25 languages. It helps doctors summarise examination history and highlight differences between reports and medical images. Besides, it provides personalised Electrical Medical Records Analytics and smart EMR search. For hospital operators, EDS delivers real-time insights through intuitive multi-dashboards and AI predictive analysis, empowering data-driven decisions for resource allocation. The excitement extends to DrAid™ Copilot - a medical AI-powered virtual assistant that helps healthcare professionals look up, extract data, and enhance productivity through natural language interaction. All the EDS modules are organised under the secure and centralised Data Lake and Data Management infrastructure, converting data to structured formats, and massive silos into a repository for world-class security and lasting and centralised storage for operative and R&D purposes. Oncology D&T, on the other hand, proves a broader adoption of AI to more accurate diagnosis and improved treatment decisions for the most complex diseases of mankind’s history. VinBrain is enlisted among the few pioneers in the world to develop a screening and treatment-aiding platform to empower the fight against liver and rectal cancer, two of the top 10 deadliest cancers in the world. The innovative AI-powered solutions, CT Liver Cancer D&T and MRI Rectal Cancer D&T reveal the intricacies of cancer classification, localisation, and measurement, enabling early detection and offering hope for saving more lives. Notably, CT Liver Cancer D&T can detect hepatocellular carcinoma (HCC), the most common liver malignancy. This solution utilises a novel method based on wavelet radiomics features from multiphase CT images for HCC screening. The study of this method was recently published in Nature Scientific Reports just ahead of RSNA 2023, coinciding with CT Liver Cancer D&T advancing to the final round of the ASEAN Digital Innovation 2023 Awards. The Oncology D&T utilises all the SOTA, including the nnUnet, ConvNeXt, and attention techniques. It is trained on and validated in high-quality and large datasets, confirmed by experienced radiologists. By providing comprehensive information, it aids oncologists in weighing treatment and surgery options such as surgical resection and transarterial chemoembolization (TACE), and total mesorectal excision (TME). Oncology D&T aim is to extend the life expectancy for a healthier life for patients and work as a second reader, while is beneficial to improve the workflow triage. Guided by a quality-first approach throughout our 4-year journey, VinBrain prioritises our strategic collaboration with esteemed institutions like Stanford University. As a guest speaker, Dr. Michael C. Muelly, Clinical Assistant Professor of Radiology at Stanford University, VinBrain’s Medical Partner shared: “I enjoyed spending time on the product launch. It's great to see what VinBrain is working up, very excited to see what is coming. The opportunities for AI to make big impacts around the world and medicine are huge. Viet Nam could be the place where it happens, starts, and spreads from there.” DrAid™’ can be easily integrated and deployed into many systems (PACS, HIS, RIS, EMR...), with the option of cloud or on-premises availability through the DrAid™ Appliance. VinBrain's team collaborates with NVIDIA biweekly to take advantage of NVIDIA's 48 GPUs, besides MONAI, and Tensor RT. DrAid™ adheres to HIPAA and NIST standards by employing Azure, ensuring robust privacy and security. At RSNA 2023, VinBrain also showcased its commitment to providing accessible healthcare in underserved regions with high tuberculosis burden through DrAid™ for Tuberculosis Screening (CXR Screening). This cost-effective solution enables large-scale screenings for an estimated 10.6 million TB-suspected individuals, garnering substantial praise from other exhibitors. Continue to learn and thrive! As Artificial Intelligence continues to make strides in radiology, such participation is a valued experience for VinBrain in every aspect including research and development, innovation and commercialisation capacity. With in-person fruitful discussions with nearly 500 visitors for four days, as well as witnessing 670 industry leaders at the Exhibits, VinBrain and its flagship product - DrAid™ has gained a lot of knowledge to reinforce the global expansion strategy and are poised to make a significant impact in the SEA, US, India, UK/EU, and Middle East markets between 2024 – 2027. About VinBrain VinBrain is a start-up that has been operating for four years and is backed by Vingroup, the largest conglomerate in Viet Nam. The company's mission is to integrate AI and IoT technologies to enhance people's lives and productivity. With an impressive portfolio of cutting-edge tech products and platforms, such as DrAid™ and AIScaler™, VinBrain has developed more than 300 AI models specifically designed for processing medical images. These models have been built using a dataset that comprises over 3.6 million images and extensive text-based big data from Viet Nam, the USA, India, China and Europe. Collaborated with leading organisations, institutes & and prestigious hospitals in Viet Nam and the United States, VinBrain's notable achievements include the deployment of DrAid™, a comprehensive AI platform for diagnostic radiology and healthcare management, in over 175 hospitals across Viet Nam, Myanmar, New Zealand, India, and the USA. For more information, press only: VinBrain JSC, info@vinbrain.net About RSNA RSNA® has over 48,110 members in 160 countries. The RSNA Scientific Assembly and Annual Meeting is the premier annual radiology forum in the world. It has been held consecutively in Chicago since 1985. The Technical Exhibits – AI Showcase within the framework of RSNA 2023, which occupied the North and South Hall of McCormick Place of 396,000 square feet and greatly immersed with 670 leading manufacturers, suppliers, and medical information and technology developers. It is the world’s largest scientific, educational, and commercial encounter for the wide array of latest healthcare innovations, and research in medical imaging, including artificial intelligence (AI), 3D printing, CT, MRI, and more. Contact Details VinBrain Phuong Nguyen, Marketing Manager +84 98 304 66 50 phuong.nguyen@vinbrain.net
December 04, 2023 08:30 AM Eastern Standard Time
Image